Well, I worked 9 hours today. And now I’m back at work, but I’m making the best of it! I’m working to fix a machine that is not responding correctly, and it takes a lot of time to run each test, so I’ll blog in between. Someday I’ll talk about life as a scientist because I don’t think I KNOW that people have no idea what kinds of things I call work.
But, back to sugars….Carbs II….or ‘sweeteners’ as not everything will be a carbohydrate in this post (bonus points if you pick out the non-carbs!).
So, there are a multitude of natural and man-made sweeteners: Honey, Maple Syrup, Agave Nectar, Brown Rice Syrup, Stevia, Splenda (sucralose), Aspartame, and sugar-alcohols like Sorbitol or Mannitol. In this anti-carb climate, each claims to be better tasting, healthier, or more natural than others. I’m going to break down the science of these sweeteners and give a couple of opinions on them. WARNING: I’m building off of the last science post: Carb I, so if I confuse you, you could scan through the old info and see if it becomes clearer.
Honey, Maple Syrup, Agave Nectar, Molasses, and Brown Rice Syrup are all naturally derived sweeteners sold in the US. They vary somewhat by carbohydrate and water content, but are all calorically similar. Check out this sweet graph I made for you guys:

The code in the carb column is which sugars each sweetener contains.
G = Glucose
F=Fructose
S=Sucrose (Glucose + Fructose)
M = Maltose (Glucose + Glucose)
Mt= Maltotriose (Glucose + Glucose + Glucose)
I = Inulin (Fructose X 3-4 units + 1 glucose)
So what does this mean? Well, there are a few take away messages. If glucose is the preferred carbohydrate for the brain, you probably shouldn’t be relieving low blood sugar with Inulin, meaning Agave Nectar would be a bad choice. Maybe if you’re very active and need a lot of both quickly metabolized and slowly metabolized glucose, Brown Rice Syrup, which is all glucose in a variety of polymers would be a good choice.People have been freaking out about how agave nectar has a similar nutritional profile as high fructose corn syrup (HFCS- a sugar I addressed in Carb I). It does have a similar nutritional profile - perhaps more fructose than HFCS. And I do think your body addresses fructose differently than glucose. However, to the best of my knowledge Agave Nectar is added to sweet things. It costs more than HFCS, and it isn't used as a filler in lots of foods that don't need it. I occasionally use it when I need a neutral tasting syrupy sweetener - like when I'm making a dessert or sweetening an iced coffee. The important part is that I use it to sweeten things that I don't neccesarily need nutritionally. I don't require the Agave Nectar to signal the 'full' feeling of raised blood sugar: because it has more fructose than glucose (4x!) it will not do that the same way sucrose does, but I do use it to sweeten treats. End of story.
Also, a good rule of thumb is that the darker the syrup is, the more trace nutrients it has. These vary, and I don’t really think they need to be counted towards daily nutrition values, but they might make things taste better and it might change the way the molecule acts when say, you bake it.
Ok, so let’s talk about no- or low-calorie sweeteners: Sucralose (Splenda ®), Aspartame (Equal®), Stevia (Truvia ®), and the sugar alcohols that usually are sorbitol and mannitol.
No matter what you think about eating it, Sucralose, the chemical that is Splenda was an ingenius invention. Why? Well, let's look at the chemical structures. This doesn't require a trained eye. Just tell me what the difference is:

I purposefully picked two that are drawn similarly. So, on the left is sucralose (Splenda (R)) and on the right is sucrose. They are almost the same, yet there are two chlorine atoms replacing some hydroxyl (or OH) groups in sucralose. This makes all the difference. The enzymes that break down sucrose to glucose and fructose don't recognize sucralose, so it's calorically at about zero. But your taste receptors treat it just like sugar. Amazing! Is it safe? Well, the FDA says it is, but the reality is that we won't know until it's been around a long time. However, studies were done and it appears safe by all known tests. The only thing that worries me is that when they radiolabeled sucralose and monitored excretion, not all of it was excreted. In some subjects only 85% was excreted. So, if you can't use it, where is it going?
Truvia, an extract of the Stevia plant, attempts to cut into sucralose's market by claiming to be a natural sweetener, therefore a better sweetener. So, let's demystify these claims.
1) Yes, stevia extract is from a plant. Sucralose is man-made. Stevia is therefore naturally occuring.
2) Stevia isn't just one compound. It's an extract from plants. These compounds are made by adding different sugar groups to a terpene backbone (don't know terpenes? See the terpene post to learn about how they help you see better and cure cancer!). Sucralose is one compound.
3) You don't metabolize either of them. Both are considerably sweeter than sugar, so you need to ingest only minimal amounts to get the desired sweetness.
Stevia appears safe. So does sucralose. Personally, I think stevia tastes funny. But that's all there is to it.
My mom ate so much equal when we were little. Now all you hear about is how the active ingredient, aspartame, causes cancer in rats. Aspartame is another one of those molecules that your body doesn't recognize, but this one was dreamed up in a test tube and just happened to work. It's nothing like the natural sugars in your body. It's designed to inhibit enzymes in your stomach that lead to ulcers (which was it's intended use), but before you freak out, there have been 20-30 years of chemists making drugs like that for medicine all the time. Biology is complex; it doesn't always work the way we model it and think it should. As for the safety of aspartame: obviously it's still around. It's in most diet sodas. I don't think it's fantastic for you, as I don't think telling your body you're getting calories and then not giving it those calories isn't very good. I just think that if it really causes cancer in high incidence, there should be a whole bunch of 30-50 year old women with liver cancer right about now.
Last one: Maybe you've tried those Russell Stover Sugar Free candies and given yourself a stomach ache. Wonder why?
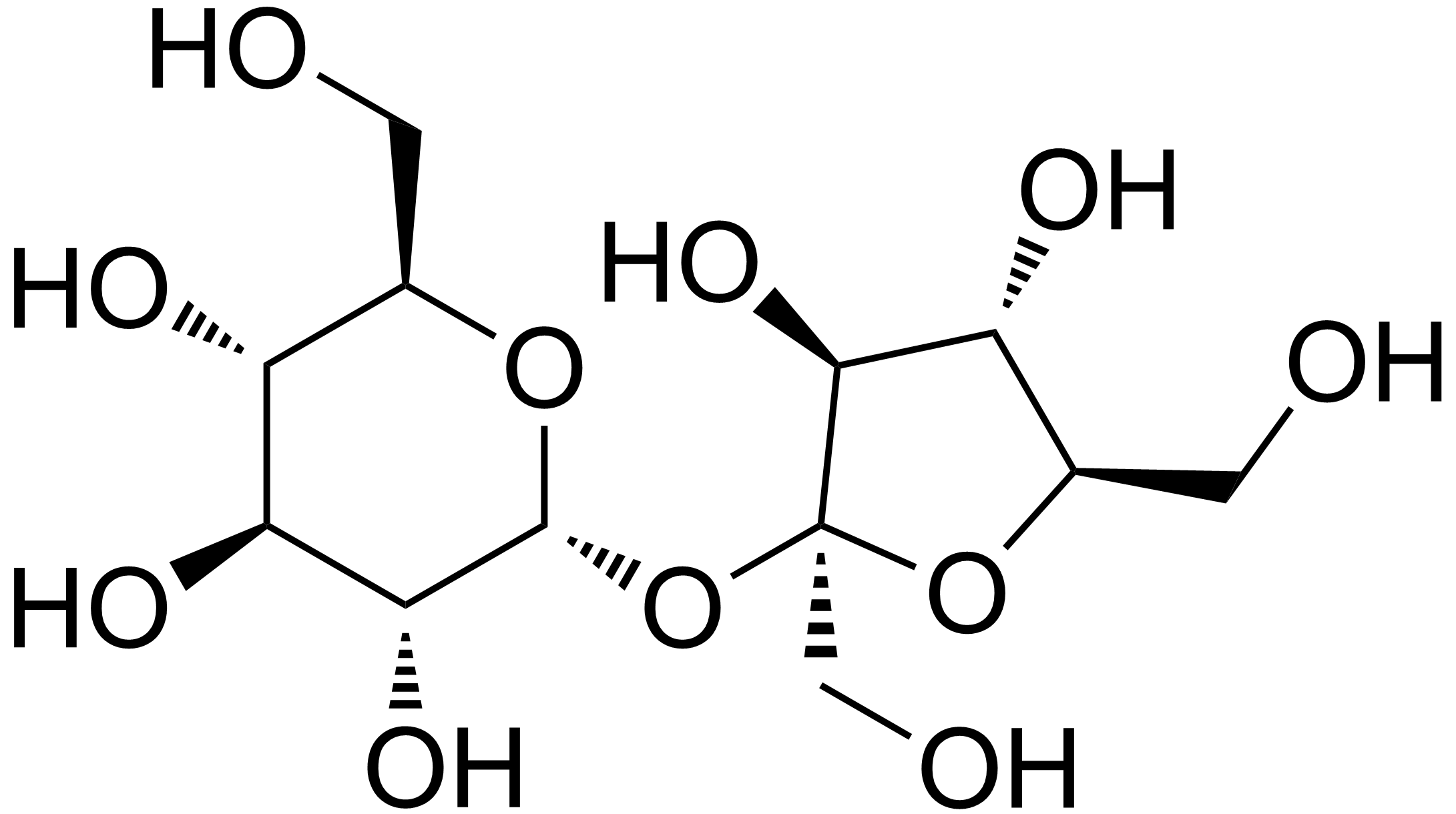
We've been talking about non-sugars for a while, but these are actually made with sugars. They are modified sugars that your body cannot digest. These are called sugar-alcohols. How do they work?
Well, if you look at sucrose, or even sucralose, you see that sugars form these pretty ring structures:
And your body recognizes these structures. The proteins that break down the sugars into energy require the sugars to be in this ring structure to metabolize them. BUT chemistry is tricky, and these structures actually go back and forth (interconvert) between a chain and a ring. So, the ring above actually looks like this chain:
This is the trick though - the sugars that are important to your diet all have the oxygen (O) with two lines to it (called a double bond) at either carbon 1 or carbon 2. That makes it so the ring can form by the -OH at carbon 5 attacking the double bond oxygen. If we replace the double bond oxygen with another -OH, this reaction can't happen. Which is exactly how sugar alcohols look:
Here is another set: Mannose (a sugar) and mannitol (a sugar alcohol):

Again, your tastebuds aren't smart enough to figure this out, but your body knows they can't use them as food.
Ok, well I'm still fighting with my equipment tonight, so I'm going to go focus on that. Back with food by Friday!











Hey lady! Another witty and interesting yet scientifically sound post. I saw this quiz and thought of you. (admittedly it is running on some pretty loose correlations to arrive at an answer - ahem, pink panther, I wonder which one that is?!)
ReplyDeleteI LOVE it. And I'm so happy to hear from you!
ReplyDelete